Specificity:>99.9%(95%CI:98.17%-100%)
Brand:
DEEPBLUEIntended use
This product is used for in vitro qualitative detection of the SARS-CoV-2 antigen in human saliva specimen. This product is intended for home self-testing as a rapid test for novel coronavirus infection. Both symptomatic and asymptomatic infections can be tested.
Test principle
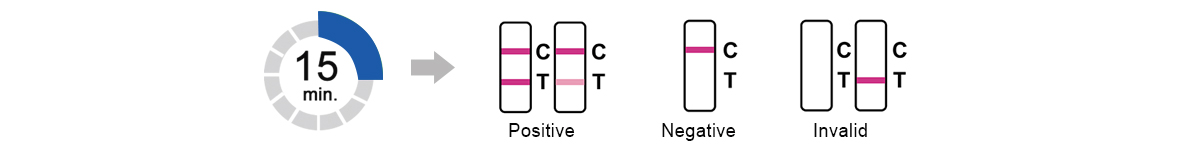
This product uses the double antibody sandwich method to detect the SARS-CoV-2 N protein. When the sample contains the coronavirus antigen, both the test line (C) and the control line (T) will appear, and the result will be positive. When the sample does not contain the coronavirus antigen or no coronavirus antigen is detected, the test line (T) will not appear, only control line (C) will appear.
Product advances
1.Pre-filled buffer solution, easier operation.
2.Passed the PEI evaluation.
3.Room temperature storage.
4.No need instrument, get results within 15 minutes.
5.Identify acute or early infection.
6.No reduction in sensitivity test against the Alpha, Beta, Delta, Gamma, Lambda, Omicron variant and so on.
Materials and Components
1.Test Device
2.Instruction
3.Antigen Extraction Tube with Extraction Reagent
4.Collection Bag
5.Sterilized Swab
6.Timer(Materials required but not provided)
Product Information
| Specification | 1pcs/box,5pcs/box,25pcs/box |
| Specimen | Human Saliva |
| Storage | 4-30℃ |
Test Procedure
Allow test device extraction reagent and specimens to equilibrate to room temperature(15 ~ 30 ℃) prior to testing.Please keep the temperature at 15 ~ 30 ℃ and the humidity at20%-80% during the whole test.
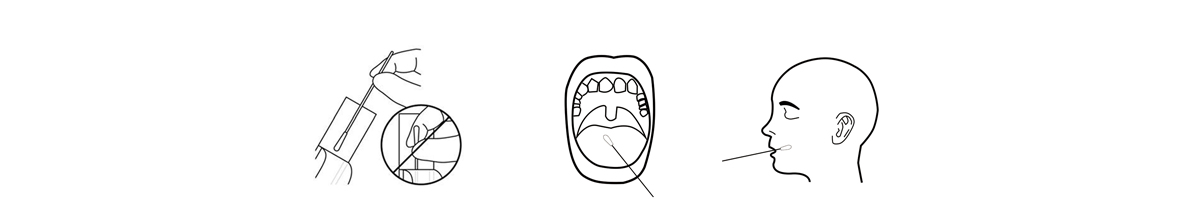
1.Specimen Collection
(1)Remove the sterilized swab from the packaging.
(2)Insert the soft tip of sterilized swab into the mouth, actively swab inside of the mouth and tongue to collect saliva forapproximately 30-60s until the soft tip
2.Testing
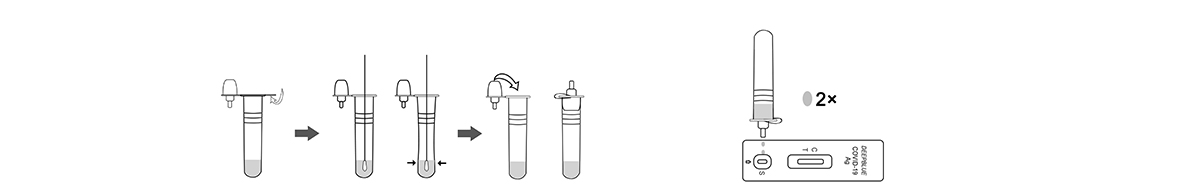
3.Test Results
Read result at 15 minutes.Do not read results after 30 minutes.
Clinical Performance
There were 600 cases overall in the study, including 150 positive samples and 450 negative samples. Statistics of nasal swab test results were as follows:
|
Reference RT-PCR Assay |
95% Wilson Score CI |
|||||||
|
LCI |
UCI |
|||||||
|
DEEPBLUE SARS-CoV-2 Ag Test |
|
POS |
NEG |
Total |
PPA |
98.7% |
92.33% |
99.07% |
|
POS |
148 |
0 |
148 |
NPA |
>99.9% |
98.17% |
100% |
|
|
NEG |
2 |
450 |
452 |
PPV |
>99.9% |
98.17% |
100% |
|
|
TOTAL |
150 |
450 |
600 |
NPV |
99.6% |
92.76% |
99.31% |
|